Experiment: Reduction of Camphor
Week 4
Now would be a great time to start reviewing
(or learning!) about NMR and IR spectroscopy. Don't leave it to
the last minute!
In the reduction of camphor, there are two possible sites of attack
by the hydride reducing agent (sodium borohydride). As seen on
p. 229 of your text, these two possible approaches are termed
exo (top face attack) and endo (bottom face attack).
It should be noted that this convention is not simply established
by which side happens to be on top or bottom. In other words,
exo does not always refer to top face approach and endo
does not always refer to bottom face approach. Consider the following
graphic:
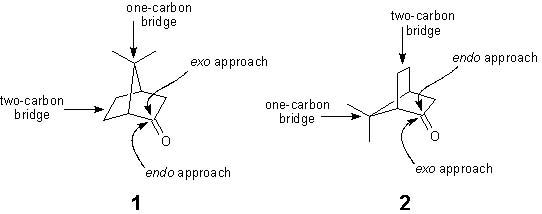
The terms exo and endo are strictly dependent on
the size of the carbon bridge nearest the side of approach (in
the bicyclic system). The substitution at the bridge carbons is
irrelevant to the assignment of the exo and endo
convention. Notice that structures 1 and 2 are simply
enantiomers of each other. Structure 2, however, is the
enantiomer of 1 rotated 120 degrees counterclockwise. Thus: